Working Time
-
Mon-Fri 09:00 - 10:00
Saturday 09:00 - 7:00
Sunday closed
Contact Info
-
Phone: 0304 1275337 (ASLEEP)
021-111-254-642 (CLINIC) - info@thesleepcentre.pk
tDCS-Transcranial Direct Current Stimulation
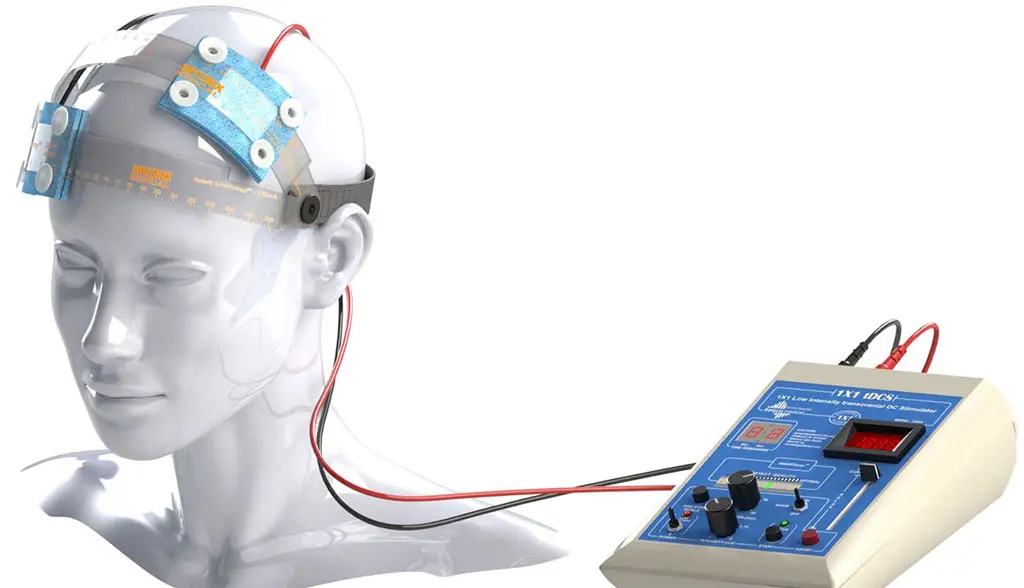
We are the first and so far only institution to bring tDCS to Pakistan.
Some clinical applications tDCS and well established for
- SLEEP DISORDERS
- DEPRESSION / ANXIETY
- OCD
- PTSD
- ADHD
- ADDICTION
So it is also it is currently being explored for schizophenia aphasia epilepsy is currently being explored for are depression, schizophrenia, aphasia, addiction, epilepsy, chronic pain (migraine, fibromyalgia), attention, and motor rehabilitation. tDCS is also used for non-medical wellness applications, for example accelerated learning, focus, relaxation, and meditation currently being perfomred by Dr. Sarosh Haque and so far very postive results.
The current delivered by tDCS is NOT strong enough to trigger an action potential in a neuron; instead its “sub-threshold” changes the pattern of already activity neurons. Think of the brain as active, trying to do or learn something, and tDCS coming along to boost this ongoing activity. At the cellular level, tDCS changes neuronal firing and by strengthening synaptic transmission between neurons by augmenting synaptic plasticity which is, in turn, the cellular basis of learning. tDCS is often combined with training. Training in itself produces learning (synaptic plasticity), and concurrent tDCS amplifies these effects (enhances synaptic plasticity).
Research on the side-effects of tDCS is ongoing, but so far the established side-effects are minor, and restricted to the electrode location. They include temporary skin redness, itching, and tingling. Other suggested side-effects of tDCS include headache, nausea, and dizziness. There is no scientific evidence that demonstrates lasting injury or irreversible side-effects from tDCS.
The FDA has provided “513g” letters to several companies explicitly allowing them to market tDCS for specific non-medical uses. The FDA also does not regulate the practice of medicine, meaning they don't regulate doctors. For this reason many doctors provide treatments that are “off-label” - things that doctors think work but do not have a “marketing” label from the FDA to the company. tDCS is approved for medical treatment in much of the world including the European Union,Israel, and Singapore. In summary while tDCS is currently not approved by the FDA, this does not mean tDCS cannot be legally tested or used in specific contexts.
The anxiety reduction effects of tDCS have been reported in several clinical trials from top medical center. Techniques related to tDCS, such as transcranial Alternating Current Stimulation (tACS) have shown promise for anxiety in clinical trials. Another related technique Cranial Electrotherapy Stimulation (CES) is FDA cleared for anxiety.
tDCS Treatment of Depression
Several clinical trials have reported tDCS can treat depression. tDCS also has much less side effects than drugs. In much of the world, including across Europe, tDCS is approved for treatment of depression.
Benefits of tDCS
tDCS is used for many different applications that involve changing the brain to influence how people think or feel. tDCS is often combined with some other form of activity or training, with the goal of tDCS boosting that specific brain activity. tDCS has been shown to make people learn faster For example, tDCS can enhance mindfulness E meditation, also seen good results in learning maths. People are also interested in tDCS to boost “working memory”.



